
BREATHING OXYGEN
Ah! Oxygen - the gas of life. Without it for a few seconds and the world disappears from view. Too much of it can be a bad thing as well.
Visiting a person receiving common oxygen therapy in a hospital refutes the notion that the oxygen is pure. A heart attack victim may be breathing oxygen through a nasal cannula Air is also entering the nose at the same time the oxygen is. Even "oxygen tents" were not totally closed. Breathing pure oxygen over a period of time may lead to serious lung irritation and even pneumonia. Breathing pure oxygen at pressures greater than 1 atmosphere can lead to fatal consequences.
Years ago there was a scuba introduced that allowed the diver to breathe pure oxygen. It was called a Rebreather. Because of the design, a small bottle of oxygen, about the size of today's pony bottles, allowed the diver to remain underwater for up to 4 hours! The unit had the appearance similar to an accordion. The air was first removed from it to flush the nitrogen out and then it was filled with oxygen. As one breathed in the bag would get smaller. As one breathed out the exhaled air would enter the bag and it would get bigger again. However, the amount of oxygen returning would be less due to the body's needs, so more oxygen would have to be added to the bag. To remove the poisonous CO2 in the exhaled air there was a canister that contained caustic Barium Hydroxide (similar to Drano). As the exhaled air passed through the canister the chemical would combine with the CO2 removing it from the breathing oxygen. So, the oxygen molecules were breathed over and over again until they were converted to CO2.
Conventional scuba is wasteful. A breath is taken, 21% oxygen goes into the lungs, 18% comes out. Little CO2 goes in and about 4% comes out. Even though there is plenty of oxygen left in the exhaled air, it is discharged into the water. Just to get rid of the CO2 we waste the oxygen. Conventional scuba is known as an "open-circuit" breathing apparatus. Oxygen rebreathers are called, "closed-circuit." Rebreathers emit no bubbles unless the diver is ascending and the bag gets too full. U.S. Navy Seals use rebreathers to avoid detection.
If a person breathes pure oxygen at a pressure above 1 atmosphere there is a possibility of it becoming toxic. Above 2 atmospheres almost insures it. Using a closed-circuit rebreather at 33' would allow the diver to breathe 2 atmospheres of oxygen. The National Oceanographic and Atmospheric Administration (NOAA) prohibits their divers from exceeding 1.6 atmospheres of oxygen. The higher the pressure, the faster the poisoning. High-pressure oxygen toxicity starts with the twitching of smaller muscles that are in use such as the ones around the mouth holding the mouthpiece. Then the twitching can lead to full-blown convulsions. Needless to say, convulsions and diving do not really go together. If fact, if you are not rescued by another diver you will probably drown. Tech divers may switch to Nitrox or pure oxygen during their dives. To protect them from drowning they wear full face masks.
The U.S. Navy Seals are a hearty lot. They have a rather rigorous training program. They must be oxygen tolerant. One of the parts to that training consists of weeding out those that convulse easily when breathing pure oxygen. Potential Navy Seals are required to sit in an air atmosphere breathing pure oxygen from a hand-held face mask at a depth equivalent to 60' of seawater for 30 minutes. If convulsions do occur they drop the mask and are quickly returned to breathing air so the convulsions will stop. If they fail the test they are booted out of the program.
Don't think you are off the hook if you dive breathing air. Air at depth can cause oxygen poisoning. At the surface air supplies 21% oxygen. Since the pressure at the surface is one atmosphere, the pressure of the oxygen alone (partial pressure) is 0.21 atmospheres. The absolute pressure at 33 feet of seawater (fsw) is 2 atmospheres, therefore the oxygen partial pressure would be 21% of 2 or 0.42 atmospheres. If you project the partial pressure of oxygen at 132 fsw it would be 21% of 5 atmospheres or 1.05. That would be the same as breathing pure oxygen at a depth of over 33'. The absolute pressure at 297' is 10 atmospheres, so the partial pressure of the oxygen would be 2.1 atmospheres and it could very well be toxic and could lead to fatal convulsions.
Since we metabolize oxygen and nitrogen causes decompression sickness, it would stand to reason that if the gas a diver breathes has a higher percentage of oxygen and less nitrogen we might be able to stay down longer and/or reduce the risk of DCS. Enriched Air Nitrox is just that. Popular blends for "Nitrox" are 32% and 36% oxygen. On EAN-36 a diver at 100' would be just like a diver on air at 75'. The US Navy Decompression Table (air) sets a 25 minute No Decompression Limit (NDL) for 100'. PADI's EAN-36 NDL is 35 minutes. Nitrox has become a popular breathing gas so divers may extend their dives at the same time reducing the chance of getting DCS. The downside is that the maximum operating depth (MOD) for nitrox is less than air because the elevated oxygen may cause oxygen poisoning. Breathing EAN-36 at 130 feet would put the diver at 1.78 atmospheres of oxygen, well over the PADI/NAUI limit of 1.4. At Deep-Six we periodically run one day courses training divers to become certified to dive with EAN.
So, what's the deal on Trimix Diving? Trimix divers have invested a lot of time in training, and a lot of money for equipment. They are able to dive to 300' or more to explore areas way out of the conventional scuba diving limits. How is this done and why so much training? The Technical Diver may be wearing 4 tanks. Two tanks on the back may contain trimix which might be a mixture of 3 gases such as 15% oxygen, 75% helium, and 10% nitrogen. The reduced oxygen is to prevent oxygen poisoning at depth, the nitrogen reduces the chance of helium tremors, and the helium reduces the narcosis caused by the other gases. On dives of less that 400' the nitrogen is not needed so the mixture might be heliox (only helium and oxygen). One of the 4 tanks will have nitrox-36 which is a "Travel Mix" to go from 90' to about 20'. Travel Mix helps to flush out the helium. The 4th tank has 100% oxygen producing rapid gas flushing for shortened decompression times. The Technical diver will start on the EAN-36 travel mix and descend to 90'. Then the diver switches to the heliox or trimix and descends to the bottom, wreck, or whatever the dive goal might be. At the end of the bottom time ascent is made to 90' and the travel mix is used to get the diver to 20' There the pure oxygen is breathed resulting in a rapid elimination of the helium and nitrogen from the tissues (Graham's Law). Because the depth of the dive was extreme the heliox or trimix supply is quickly consumed. Some Technical Divers use very sophisticated rebreathers that meter in the oxygen according to what is consumed and the percentage changes, but not the oxygen partial pressure, according to the depth. Chi-Chang!




THE NITROX COURSE

Nitrox is a mixture of gases that
should be part of every diver’s choice. To be certified to use Nitrox
you must take a course such as the PADI EAN (Enriched Air Nitrox) course offered
at Deep-Six. The gas mixture will allow a diver to remain underwater for a longer
duration than if diving with air.
Air is 78% nitrogen and 21% oxygen. Because there
is 21% O2 it could be called EAN21. If the amount of oxygen compressed into
a scuba cylinder was increased to 32% it would be called EAN32. The amount of
N2 would be decreased to 68%, 10% less than the normal amount. How would that
affect the dive?
USE THE 3 TABLES: The RDP, the EAN32, and the EAN36
1. What is the No-Stop (No Decompression Limit NDL) limit for air at 62'?
2. What is the No-Stop (No Decompression Limit NDL) limit for EAN32 at 62'?
3. What is the No-Stop (No Decompression Limit NDL) limit for EAN36 at 62'?
Looking at the PADI RDP (EAN21) the No-Stop Limit
for 100’ is 20 minutes. If you were breathing EAN32 that chart shows the
limit to be 30 minutes. On EAN36 the limit is 35 minutes. That extra 15 minutes
would be quite an increase in the dive time. Because the amount of nitrogen
being absorbed by the diver is less the chance of Decompression Sickness (DCS)
is less as well. So then, it would seem if we breathed EAN100 the chance of
DCS would be zero. That sounds great. Unfortunately oxygen has some serious
side affects preventing divers from doing that safely.
It would seem breathing increased partial pressures
of oxygen would prevent Nitrogen Narcosis and we would be less intoxicated.
It has been found oxygen produces narcosis as well when diving deep so Nitrox
will not provide any benefit. When Nitrox first arrived on the diving seen many
divers claimed they felt really good after the dive. A double blind study proved
that was mainly psychosomatic.
There are 2 serious issues with breathing higher
levels of O2: Pulmonary/CNS OxTox and Partial Pressure OxTox. Pulmonary OxTox
is caused by breathing too much oxygen over a long period of time and Partial
Pressure OxTox is caused by breathing high partial pressures of oxygen over
a short period of time. Either may result in death. Each of us has a certain
level of tolerance to breathing high levels of oxygen. Oxygen Tolerance Units
(OTU’s) help to define this. Breathing pure oxygen for 1 minute equals
1 OTU. Breathing air (EAN21) for 1 minute would equal 0.21 OTU. How many OTU’s
do you breathe every day?
Minutes in a day (60 X 24) = 1440
OTU’s you would acquire if you breathed air for 1 day (1440 X 21%) = 302
OTU’s you would acquire if you breathed pure oxygen for 1 day (1440 X
100%) = 1440
The maximum limit for OTU’s is about 850 per day.
If you were in the cardiac unit of a hospital
and were placed on pure oxygen your daily limit would be exceeded. The longer
your body was exposed the more the chance Pulmonary/CNS OxTox would occur. Lung
and throat irritation with breathing difficulty, a cough, perhaps some wheezing,
and inspiration may become quite painful. Death would be possible due to the
lung assault leading to pneumonia thus preventing O2 getting to the blood. When
being administered oxygen in a hospital the delivery is usually with a nasal
cannula and those put into your lungs about 60% oxygen reducing the chances
of Pulmonary/CNS OxTox.
Underwater a diver on Nitrox or air is exposed
to higher than normal oxygen (OTU) levels because of the increased depth pressure:
Air at sea level = 302 OTU per day
Air at 33’ (2 atmospheres) = 604 OTU per day
Air at 66’ = 906 OTU per day
At 66’ that’s over the 850 OTU limit. If you were in that environment for several days Pulmonary/CNS OxTox systems might appear. But then, how many divers would be that deep for that long. Things could change if the mixture of gases changed. A diver breathing pure oxygen during decompression could get Pulmonary/CNS OxTox if they had to repeat the treatment more than one time.

60’ for 20 minutes = 60/33 +1 = 2.82 atm = 56.4 OTU
(pressure lessened) 45’ for 30 minutes = 45/33 +1 = 1.75 atm = 52.5 OTU
30’ for 120 minutes = 30/33 + 1 = 1.91 atm = 115 OTU
15’ for 30 minutes = 15/33 +1 = 1.45 atm = 44 OTU
The total CNS exposure would be 268 OU.

The DSAT Table is your way of preventing Pulmonary OxTox. You are limited to 100% in a 24-hour period. Further information is below.
High Pressure Oxygen Toxicity – Partial Pressure OxTox (from Brylske’s “Oxtox”). This is classically known as “O2 Poisoning” in scuba diving.
Dalton’s Law of Partial Pressures needs a little review: Air: 78% N2 and 21% O2 sea level. The pressure there is 14.7 psi so:
LOCATION TOTAL PPN2
PPO2 ON
PURE O2 psi
SURFACE: 14.7psi
11.47psi 3.09psi 14.7psi
ROUNDING: 15
12
3
15
33’:
30
24
6 30
66’:
45
36 9
45
132’:
75
60
15 (14.7 psi) 75
Using air at 132’ has the same O2 as pure O2 at the surface. It is 1
atm. (But that does not exceed 1.4 atm O2.) Exceeding 1.4 atm of oxygen may
cause Partial Pressure OxTox. Underwater it could be more deadly than Pulmonary/CNS
OxTox and can happen without a significant warning.
PADI and NAUI maximum PPO2 is 1.4, NOAA’s is 1.6 atm.
PADI Contingency Depths (PPO2 = 1.6 atm) What should you do if you enter the
Contingency Zone? (Ascend above 1.4 ata O2 immediately and then end the dive.
Do not make a repetitive dive for 24 hours.)
Using the PADI DSAT Equivalent Air Depth Table, look at the depths, EAN mix, and O2 pp.
Using the PADI DSAT Equivalent Air Depth Table, look at the depths, limits and Contingency.
Using the PADI DSAT Equivalent Air Depth Table, look at the actual depth and the Equivalent Air Depth.
EAD = (1 - O2%) X (Depth + 33) / 0.79 -33 Figure the EAD for 60'
All blends of Nitrox have a Maximum Operating Depth. What is the MOD for EAN37?

MOD Depth Formula = (1.4/ O2% - 1) X 33
Contingency Depth Formula = (1.6/ O2% - 1) X 33
At what depth would you reach 1.4 atm of O2 on air?
1.4/0.21 = 6.67 - 1 = 5.67 X 33' = 187' is the MOD
What is the MOD for NOAA breathing pure O2? (Remember NOAA considers 1.6 to be their MOD. PADI states 1.6 is contingency.)
1.6/1.00 - 1 = 0.6 Atm X 33' = 19.8'
When a diver breathes 1.6 Atm O2 and up it my become a brain problem. There is an individual tolerance. (See a Bell Curve 34/14/2). There is an inhibition of a brain enzyme. The high levels of O2 inhibit production of gamma-aminobutyric acid (GABA) and that is a neurotransmitter that inhibits muscle reaction. Without it the neurons fire uncontrollably. An O2 hit is similar to a Grand Mal seizure. There may be NO warning! From the time a diver might realize the 1st symptoms, such as twitching muscles that are used like the lips, there may be as little as 10 seconds before the uncontrollable seizure begins. The mouthpiece will probably be spit out and unconsciousness occurs. Usually the diver will die unless rescued by another.
Oxygen toxicity (VENTID) Symptoms:
Visual Disturbance
Ears Ringing
Nausea
Twitching (leading to fatal convulsions)
Irritability, restlessness, euphoria, anxiety
Dizziness
The seizure starts with immediate loss of consciousness and 30 seconds of muscle
relaxation. Initially they will be holding their breath – lung over-expansion
is possible if ascent is made! That is followed by all of the body’s muscles
contracting violently for about 1 minute. Then there is rapid breathing and
extreme confusion when regaining consciousness.
Recall that the total Oxygen exposure allowed
in 24 hours is 850 OTU. OTU = Whole body, long exposure and can lead to Pulmonary/CNS
OxTox. The DSAT Table measures this exposure. Surface Intervals do not count
– Exposure based on 24 hours. The percentage of allowable O2 per 24 hours
on the DSAT table is the CNS exposure.
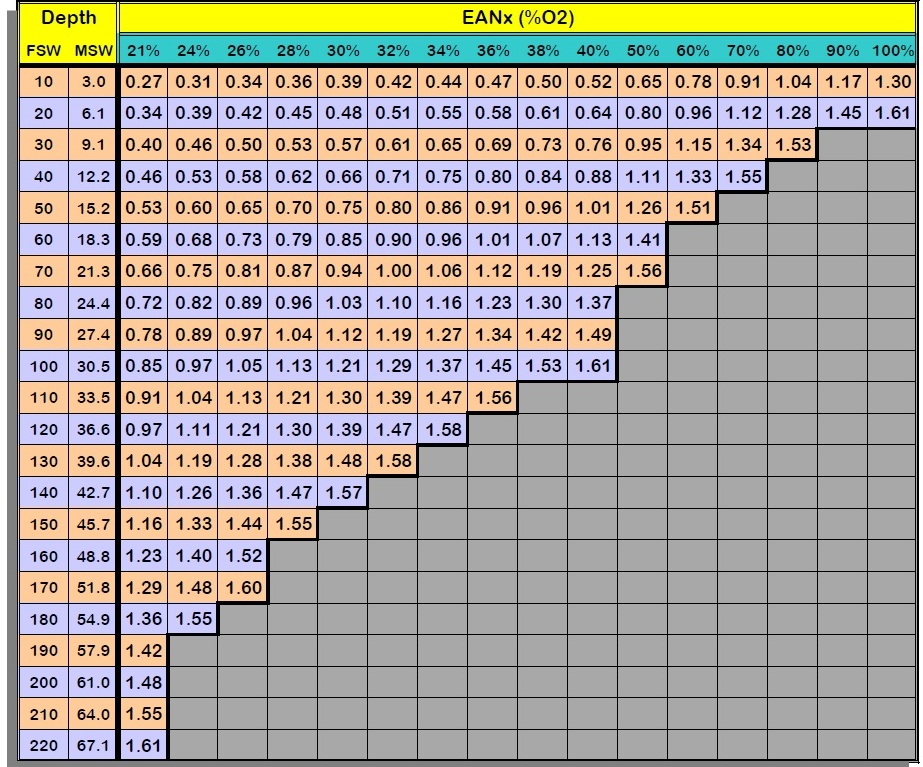
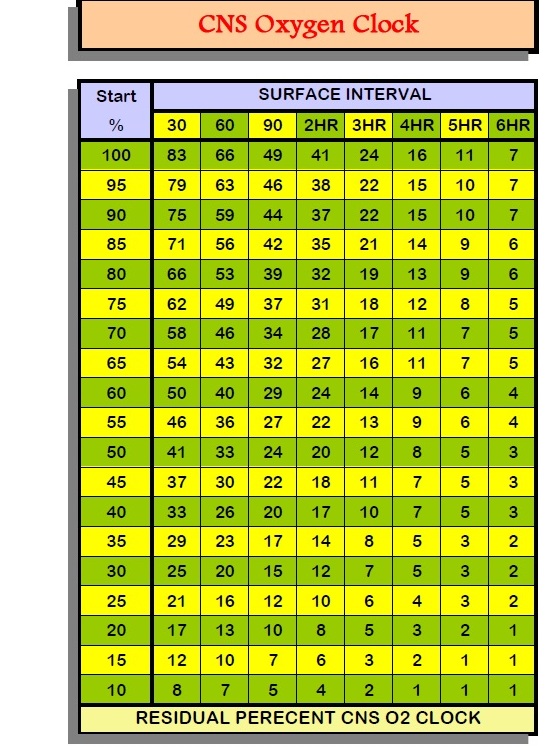
What’s your CNS oxygen exposure from the
2 dives? (1.56 for 12m and 1.23 for 25m)
Looking at the DSAT Table, if you are in Contingency during the 1st dive –
what should you really do? (rise above 1.4 ata immediately, start ascent to
surface, and do not make the repetitive dive.)
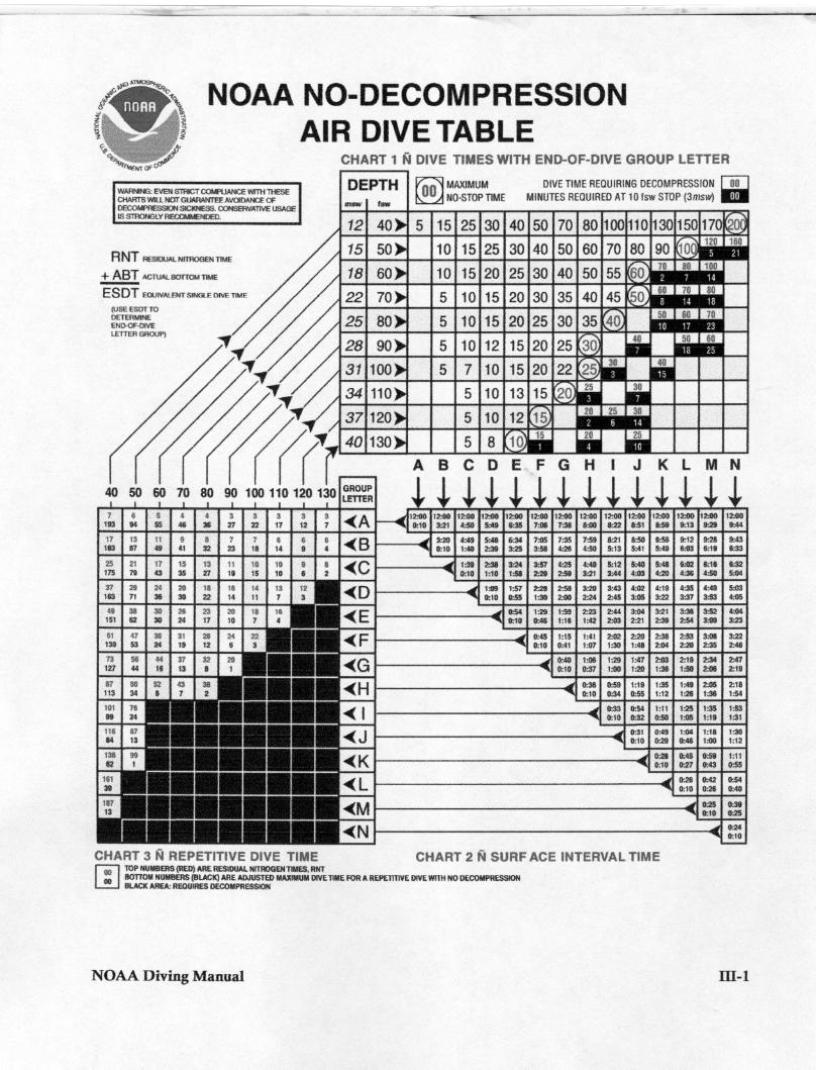

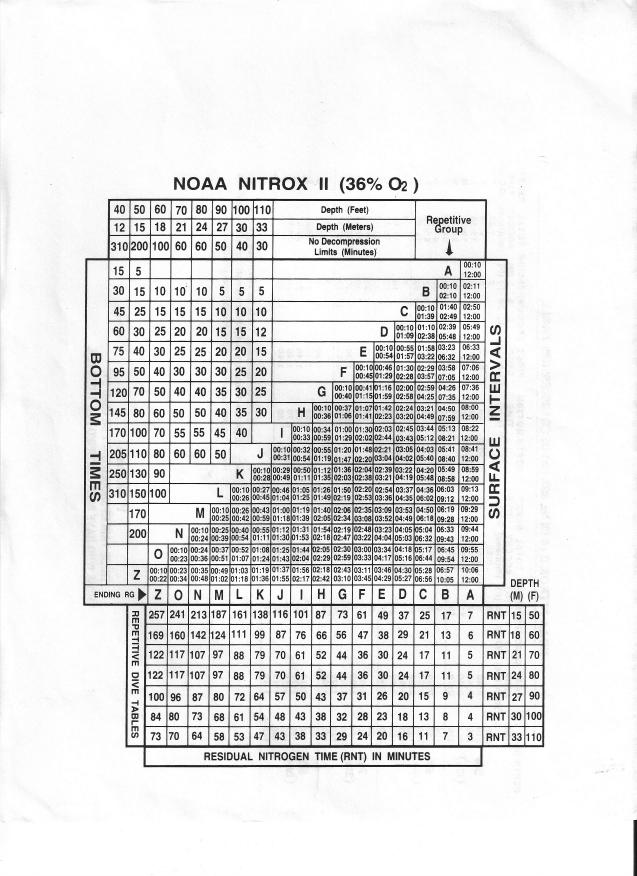
Decompression Comparisons:
Air: 115’ 12 m RG? (J)
SI = 38 min RG? (E)
52’ for 17 min: RNT? (17) RG? (N)
EAN32: Same profile (GCI)
EAN36: (Beyond Contingency)
Navy Table: (EEH)
The percentage of allowable O2 per 24 hours on the DSAT table is the CNS exposure.
CNS (Above EAN32 Dive)? (Dive 1: 10%; Dive 2: 5%; Total 15%)
CNS EAN36 = X
CNS EAN34 = X
CNS EAN30: (10% and 5%)
Five considerations when dealing with Nitrox:
1. O2 Toxicity (Oxtox) – CNS + OTU
2. Special equipment needed such as a dedicated cylinder, O2 analyzer
3. Availability
4. Gas blending and handling
5. More planning and more potential for error
You can see the pp of O2 in your scuba tank is critically important. When you get a tank on a boat, from a dive shop, or from your friend you must analyze the oxygen content. Then you must mark the tank with your name and the O2 content. Then you must determine the MOD to avoid going into Contingency or beyond. Then you must calculate the CNS exposure you received during the dive and add that to any previous dives made in 24 hours. Show a EAN tank. Have them smell the Mix. We are off to the compressor station to see how Nitrox is made, analyzed, and labeled.
Points Covered:
Below 40% is safe for regular scuba equipment. Explosion dangers are present.
Non-O2 cleaned cylinders and regulators are safe for up to EAN40
Partial Pressure vs Continuous Blending: Demonstration of Continuous Blending and Analysis with completion of tank decal.
O-rings in the tank neck.
Christolube.
Return to class with EAN tank for student analysis. Fill out the EAN Tank Decal with the EAN, MOD, pressure, and name.
EAN32 is also known as “Nitrox I”
EAN36 is also known as “Nitrox II”.
EAN may be used as First aid for cardiac, decompression (e.g.) patients. Do
not use the purge Valve
Rescue for a diver convulsing:
Hold the mouthpiece in, or leave it out if it is out.
Surface safely – convulsing divers may hold their breath.
Get buoyancy
Check for airway and breathing
Call for assistance
Transport, O2, Compressions if needed
First Aid – ABC’s (Breathing in water only, no rescue
breaths after leaving the water)
Problems:
Problem: EAN32, what’s the EAD for 70’? (56’) – Now use the RDP for the dive.
Problem: EAN36, EAD for 76’? (59’)
Problem: EAN40, EAN for 53’? (38’)
Problem: EAN37, EAN for 70’? (49’) NDL for this dive? (80 minutes)
Problem: EAN30, NDL for 103’ (20m)
Problem: EAN31. 75’ for 37m followed by a SI of 1h 15m. What are the
NDL and RNT for the 2nd dive?
(59m NDL, 21m RNT) The Repetitive Group would be? (E)
What if you wanted a nitrogen flush on the 2nd dive and used EAN40?
What would be your total oxygen exposure (CNS) if you dove to the new Adjusted
NDL?
Exercise 3 (page 20)
Exercise 4 (page 26)
Knowledge Review (page 34)
THE FINAL EXAMINATION: CLICK HERE: (The Exam Link will be back next class.)